CAR-T Cell Therapy Market to Surpass $20.4B by 2033, Fueled by Oncology Breakthroughs and R&D Push | DataM Intelligence
The CAR-T Cell Therapy Market is growing rapidly with expanding indications, rising trials, and innovations in solid tumor and autoimmune applications.
CAR-T cell therapy is redefining cancer treatment transforming immune cells into powerful, targeted therapies that offer hope where traditional options fall short.”
NEW YORK, NY, UNITED STATES, June 20, 2025 /EINPresswire.com/ -- Market Overview - — DataM Intelligence
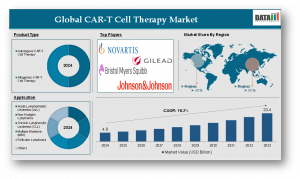
Chimeric Antigen Receptor T-cell (CAR-T) Therapy is one of the most revolutionary advancements in oncology, offering a personalized immunotherapy approach that reprograms a patient’s T-cells to recognize and attack cancer cells. This cutting-edge treatment has already transformed the outlook for certain hematologic cancers and continues to show promise in treating solid tumors and autoimmune conditions. As of 2024, the global CAR-T cell therapy market reached a valuation of US$ 4.8 billion, and it is projected to grow significantly, reaching US$ 20.4 billion by 2033, growing at a CAGR Value of 16.3% during the forecast period 2025 to 2033.
Get Latest Sample Report : https://datamintelligence.com/download-sample/car-t-cell-therapy-market
Market Drivers are :
Increasing incidence of hematological malignancies such as leukemia, lymphoma, and multiple myeloma is driving treatment demand.
Robust pipeline of CAR-T cell therapies under clinical investigation for solid tumors and autoimmune disorders.
Favorable regulatory landscape, with numerous fast-track and breakthrough designations granted by the FDA and EMA.
Growing number of strategic partnerships and licensing deals between biotechnology firms and large pharmaceutical companies.
Ongoing innovations in cell processing, gene editing, and scalable manufacturing technologies are accelerating development.
CAR-T therapies demonstrate high efficacy in relapsed or refractory cancer patients unresponsive to conventional treatments.
Key Players in the market are :
Leading the charge in innovation and commercialization of CAR-T cell therapies are the following companies:
Novartis AG
Gilead Sciences, Inc.
Bristol Myers Squibb Company
Johnson & Johnson
Autolus Therapeutics
Pfizer Inc.
BioNTech SE
Merck KGaA
Allogene Therapeutics
Atara Biotherapeutics
AstraZeneca
Eli Lilly and Company
JW Therapeutics
These key players are actively involved in clinical trials, regulatory filings, and expanding access to next-generation CAR-T products across global markets.
Market Segmentation
By Indication
Acute Lymphoblastic Leukemia (ALL)
Diffuse Large B-cell Lymphoma (DLBCL)
Multiple Myeloma
Chronic Lymphocytic Leukemia (CLL)
Solid Tumors
Others
By Target Antigen
CD19
BCMA
CD22
Others
By End User
Hospitals
Cancer Research Institutes
Specialty Clinics
By Region
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Latest News of USA -
In March 2024, the FDA approved a new CAR-T therapy developed by Bristol Myers Squibb targeting BCMA for relapsed multiple myeloma, marking it the third such approval in the U.S.
Gilead Sciences announced the launch of a next-gen CAR-T manufacturing platform to reduce turnaround time from 15 days to under 7 days.
Autolus Therapeutics received a $100 million NIH grant to expand its research into CAR-T therapies for pediatric leukemia.
Latest News of Japan -
In February 2024, JW Therapeutics announced a partnership with Japan’s National Cancer Center to launch a CAR-T trial for solid tumors.
Japan’s Ministry of Health, Labour and Welfare (MHLW) streamlined its regulatory approval process for cell therapies to support local and international innovators.
Takeda Pharmaceuticals launched a pilot CAR-T manufacturing facility in Osaka to address growing regional demand for hematologic cancer treatments.
Recent Key Developments are :
Novartis launched a Phase III trial for its new CD22-targeted CAR-T therapy aimed at relapsed pediatric ALL patients.
Pfizer expanded its partnership with Autolus to co-develop novel CAR-Ts for autoimmune disorders, including lupus and Crohn’s disease.
BioNTech SE announced successful preclinical results for an off-the-shelf, allogeneic CAR-T therapy, which will enter Phase I trials in late 2024.
Johnson & Johnson acquired a mid-stage biotech focused on CAR-T solutions for solid tumors, bolstering its oncology pipeline.
Eli Lilly made a strategic entry into the CAR-T therapy space by investing $500 million in advanced cell therapy research and establishing a new manufacturing facility in North America.
Conclusion ;
The CAR-T cell therapy market is undergoing a dynamic expansion phase, driven by strong clinical results, regulatory support, and a growing number of approved indications. As therapies transition from hematologic cancers to solid tumors and autoimmune conditions, the field is poised for continued growth. Challenges such as cost, manufacturing scalability, and access remain, but advancements in allogeneic platforms and automation are promising solutions. With the U.S., Japan, and Europe leading in clinical and regulatory progress, CAR-T therapy is shaping up to be a cornerstone of personalized oncology in the decade ahead.
Global CRISPR Market
Cell and Gene Therapy Market
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.